Capabilities & Expertise
Areas of Expertise
| Microfluidic experiments & design | Transport & interfacial analysis | Soft lithography fabrication |
| Droplet-based strategies | Interfacial tension measurement | High speed video microscopy |
| Uniform emulsion formation | Interfacial rheology measurement | Elongational & shear rheology |
Instruments and Tools We Have Built
We have developed and/or built several instruments, including a microtensiometer, a pendant bubble apparatus with code for shape-fitting that uses a non-gradient based algorithm, a linear translation microscale shear cell, and a filament thinning rheometer. More about these instruments is available on the Facilities page. We welcome new opportunities to contribute our capabilities and instrumentation to new collaborations.
The Microtensiometer
(in collaboration with Lynn M. Walker)
As part of our larger effort to quantify and control microscale interfacial phenomena [1-18], we have developed a microtensiometer device capable of measuring dynamic and static interfacial tension and interfacial elasticity at fluid/fluid and fluid/air interfaces. The novelty of the device comes from the focus on highly curved, micron length scale drops and bubbles; a length scale that allows for more rapid analysis, smaller sample volumes and the potential for parallelization. Along with the device itself, a paradigm has been developed to properly separate and characterize different phenomena in the problem (adsorption/desorption kinetics, diffusion and convection). This paradigm, in the form of relatively simple but powerful scaling arguments, allows us to design experiments and analysis appropriately for a given surface active species. This allows us to extract accurate parameter values that can be applied to models for more complex and realistic interfacially driven systems.
Advantages of the Device
The prototype device is capable of streamlining measurements of equilibrium and dynamic interfacial tension as well as interfacial elasticity of complex molecules at air/fluid or fluid/fluid interfaces. The simplicity of the device should not disguise the advantages it offers over other standard techniques (maximum bubble pressure, pendant drop, spinning drop, and others) and the fact that this technique can be easily parallelized for application to complex systems with large parameter spaces. A summary of the key advantages of the microtensiometer are:
• Small sample volumes ( < 5 mL)
• Rapid experimentation times (sec-min)
• No need for complicated shape algorithms in image analysis
• Measurement of interfacial elasticity simplified
• Potential to decouple kinetics & diffusion
Limitations of the current prototype involve user friendliness, sample environment control and the serial nature of experiments. All of these limitations can be addressed and all are solvable. The advantages of the device – small volumes, faster experiment times, and algorithm-free analysis – allow for examination of fundamental issues in colloidal systems, which are problems of enormous practical importance in a variety of industries.
Systems Studied
The list below provides a synopsis of the variety of systems that have been studied and that are currently being investigated with the microtensiometer. While many of these types of systems have been characterized using other techniques, our approach simplifies the process and enables more rapid analysis of valuable samples, potentially in a high throughput manner. This list is just a suggestion of the many complex interfacial systems that can be examined with this apparatus.
• Nonionic surfactants at air/water interfaces – a fundamental and proof-of-concept study demonstrated scaling and timescale arguments. [10, 11, 19]
• Nonionic surfactants at oil/water interfaces. Measured kinetic parameters without need for unphysical description of diffusion. [8]
• Polymer-grafted nanoparticles at air/water interfaces – investigated dynamic surface tension and interfacial mechanics for PDMAEMA-coated silica nanoparticles.[20]
• Peptides at air/water interfaces – investigating dynamics and static surface tension of short-chain peptides (23-mer) engineered to undergo conformational change at interface.
• Synergistic nanoparticle adsorption for stabilization of inverted emulsions – understanding interfacial dynamics and mechanics with regard to emulsion stability. [21]
• Polyelectrolyte-surfactant aggregate adsorption at air/water interfaces – characterization of dynamic surface tension to elucidate nanostructure adsorption mechanism.
• Competitive adsorption of surfactants and biosurfactants at oil/water interfaces – an initial study to determine the impact of synthetic dispersants on oil-eating bacterial processes.
The Apparatus and Analysis Method

Fig. 1. Schematic diagram of the microtensiometer apparatus including convection. Parts include (A) microscope condenser, (B) peristaltic pump, (C) microtensiometer sample cell, (D) pressure transducer, (E) 3-way solenoid valve, (F) constant pressure head, (G) microscope objective for image analysis, (H) 21-gauge needles and (I) micropipette.
The prototype microtensiometer is shown schematically in Fig. 1. The device is simple and robust. Glass micropipettes are used to generate bubbles with radii ranging from 10 to 200 microns. Pipettes manufactured from borosilicate stock can be purchased in a range of sizes, or produced in house using a standard micropipette puller, allowing for a wide range of sizes. During an experiment, the micropipettes are placed into a vessel filled with a small volume of the test solution. Care is taken to avoid pre-wetting of the inside of the pipette by silanizing the glass surfaces and rendering them hydrophobic (This procedure has also been adapted for generating oil drops in aqueous solution). Several bubbles are ejected from the micropipette tip prior to beginning measurements to deplete the interface of surfactants and ensure a clean initial interface, further indicated by initial surface tension values that are comparable to expected clean interface values. A window above the surfactant solution-filled reservoir allows the micropipette tip to be imaged through an inverted bright field microscope. The bubble radius is measured from these images using edge detection methods in MATLAB. Part of the simplicity of this technique arises from the fact that the interface being studied is a hemispherical cap and complex shape analysis is not needed to extract an interfacial tension.
The basis of the device arises from calculations and predictions that show adsorption is a strong function of curvature for micron length scale bubbles and droplets. [2, 22] Our results confirm that dynamic surface tension does indeed evolve more rapidly for smaller bubbles. This observation allows us to speed up the analysis by using smaller capillaries and also demonstrates the complexity associated with dynamic surface tension measurements. We have fully characterized the impact of concentration and bubble size on dynamic surface tension measurements for simple nonionic surfactants at air/water interfaces. [10, 11] We can also drive flow in the sample cell, which we can use to rapidly exchange samples, to decrease the timescales for adsorption still further, or to investigate the effect of flow on adsorption processes. [19]
To create the fluid interface, the bubble is placed in contact with a constant-pressure water column, providing a simple and stable pressure source. Bubbles (or drops) in this size range are negligibly distended by gravity (i.e. the Bond number ~ 10-5) so the Young-Laplace equation reduces to the simple expression 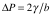 relating the pressure jump ΔP across a spherical cap of radius b to the surface tension γ. The instantaneous surface tension is determined by the measured instantaneous pressure difference across the interface, and by the instantaneous bubble radius imaged in the microscope using
relating the pressure jump ΔP across a spherical cap of radius b to the surface tension γ. The instantaneous surface tension is determined by the measured instantaneous pressure difference across the interface, and by the instantaneous bubble radius imaged in the microscope using  . Another advantage of moving to smaller bubbles is that ΔP is increased into a range that is measureable with off-the-shelf transducers. For a 10 µm air bubble in water, the gage pressure inside the bubble is ΔP ≈ 2.0 psi. This provides a measurable and controllable force that normally cannot be utilized in these experimental approaches. The microtensiometer yields static surface tension values of 72.81 ± 0.05 mN/m for de-ionized water at 20°C. The main source of uncertainty is in the imaging and measuring of micron-scale radii, and this is not a hindrance for work with intermediate sized capillaries (30 – 100 μm).
. Another advantage of moving to smaller bubbles is that ΔP is increased into a range that is measureable with off-the-shelf transducers. For a 10 µm air bubble in water, the gage pressure inside the bubble is ΔP ≈ 2.0 psi. This provides a measurable and controllable force that normally cannot be utilized in these experimental approaches. The microtensiometer yields static surface tension values of 72.81 ± 0.05 mN/m for de-ionized water at 20°C. The main source of uncertainty is in the imaging and measuring of micron-scale radii, and this is not a hindrance for work with intermediate sized capillaries (30 – 100 μm).
We note that by fixing the pressure in this experiment, we allow the radius of the bubble to change as the surface tension evolves. We computed the concomitant surface area change for the spherical cap formed by the bubble interface, and found that the exposed surface area typically increases by no more than 2-3%, similar to area changes observed in pendant bubble experiments.[23] Furthermore, we validated via numerical simulations incorporating both radius and surface area changes that the impact of the changing radius on the evolution of surface concentration and surface tension is negligible.[11]
The current experimental setup is similar to a micropipette design reported previously.[24] In that work, the authors focus on the advantages of using small sample volumes to characterize static and dynamic interfacial properties of lipid systems. Our current apparatus differs in several respects in terms of geometry; they keep their interface confined within the body of the pipette itself, we expose the bubble to the reservoir of solution. This is significant, as confinement and depletion effects can be important near the three-phase contact line for the bubble within the pipette. However, we have maintained the advantage of small sample volumes and controlled environments.
Additional Capabilities
The microtensiometer developed at CMU is a prototype device that has served well to provide proof of concept measurements, initial results, and verification of the approach. The system is quite simple and can be adapted to include the additional control and capabilities. A summary of the current capabilities is listed below:
• Direct, rapid measurement of dynamic interfacial tension for small sample volumes and microscale drops and bubbles
• Timescale analysis to decouple transport and adsorption mechanisms
• Measurement at fluid/fluid or fluid/air interfaces
• Convection cell to reduce transport timescales, exchange samples rapidly, rapidly vary solution composition, or examine effect of flow on adsorption processes
• Temperature controlled sample cell via embedded water coils surrounding the cell
• Application of oscillating pressure head for dilatational rheology measurement
• Analysis of the importance of depletion of the bulk solution on interfacial measurements [25]
Opportunities
We welcome opportunities to contribute our capabilities and instrumentation to new collaborations. Please contact Shelley Anna (sanna@cmu.edu) if you are interested in discussing ways that our group’s expertise may be able to help you address problems of importance to you in microscale, multiphase, and/or interfacial systems.
Collaboration with the microtensiometer could include instrument development and improvement to the device at CMU for characterization of specific samples of interest. Alternately, collaboration could involve development of a simplified and specific device for deployment at an external site. This would involve consultation from the CMU team in application, usage and potential help on analysis. If you are interested in working with us, please contact either Shelley Anna (sanna@cmu.edu) or Lynn Walker (lwalker@andrew.cmu.edu).
References
- Anna, S.L., N. Bontoux, and H.A. Stone, Formation of dispersions using “flow focusing” in microchannels. Applied Physics Letters, 2003. 82(3): p. 364-366.
- Anna, S.L. and H.C. Mayer, Microscale tipstreaming in a microfluidic flow focusing device. Physics of Fluids, 2006. 18(12).
- Christopher, G.F. and S.L. Anna, Microfluidic methods for generating continuous droplet streams. Journal of Physics D-Applied Physics, 2007. 40(19): p. R319-R336.
- Christopher, G.F. and S.L. Anna, Passive breakup of viscoelastic droplets and filament self-thinning at a microfluidic T-junction. Journal of Rheology, 2009. 53(3): p. 663-683.
- Christopher, G.F., et al., Coalescence and splitting of confined droplets at microfluidic junctions. Lab on a Chip, 2009. 9(8): p. 1102-1109.
- Christopher, G.F., et al., Experimental observations of the squeezing-to-dripping transition in T-shaped microfluidic junctions. Physical Review E, 2008. 78(3).
- Link, D.R., et al., Geometrically mediated breakup of drops in microfluidic devices. Physical Review Letters, 2004. 92(5).
- Alvarez, N.J., et al., The Effect of Alkane Tail Length of CiE8 Surfactants on Transport to the Silicone Oil-Water Interface. J. Coll. Interface Sci., 2011. 355(1): p. 231-236.
- Alvarez, N.J., L.M. Walker, and S.L. Anna, A non-gradient based algorithm for the determination of surface tension from a pendant drop: Application to low Bond number drop shapes. Journal of Colloid and Interface Science, 2009. 333(2): p. 557-562.
- Alvarez, N.J., L.M. Walker, and S.L. Anna, A Microtensiometer To Probe the Effect of Radius of Curvature on Surfactant Transport to a Spherical Interface. Langmuir, 2010. 26(16): p. 13310-13319.
- Alvarez, N.J., L.M. Walker, and S.L. Anna, Diffusion-limited adsorption to a spherical geometry: The impact of curvature and competitive time scales. Physical Review E, 2010. 82(1).
- Christanti, Y. and L.M. Walker, Surface tension driven jet break up of strain-hardening polymer solutions. Journal of Non-Newtonian Fluid Mechanics, 2001. 100(1-3): p. 9-26.
- Christanti, Y. and L.M. Walker, Effect of fluid relaxation time of dilute polymer solutions on jet breakup due to a forced disturbance. Journal of Rheology, 2002. 46(3): p. 733-748.
- Lee, W., L.M. Walker, and S.L. Anna, Role of geometry and fluid properties in droplet and thread formation processes in planar flow focusing. Physics of Fluids, 2009. 21(3).
- Lee, W., L.M. Walker, and S.L. Anna, Comparison between Viscoelastic and Surfactant Dynamics in Flow Focusing Microfluidics. Macromolecular Materials and Eng., 2010. 296: p. 203-213.
- Wei, Y., S. Garoff, and L.M. Walker, Impact of fluid memory on wetting approaching the air entrainment limit. Journal of Colloid and Interface Science, 2009. 337(2): p. 619-621.
- Wei, Y., et al., Dynamic wetting with viscous Newtonian and non-Newtonian fluids. Journal of Physics-Condensed Matter, 2009. 21(46).
- Wei, Y., et al., Dynamic wetting of Boger fluids. Journal of Colloid and Interface Science, 2007. 313(1): p. 274-280.
- Alvarez, N.J., et al., Using bulk convection to approach kinetic-limited surfactant dynamics. Journal of Colloid and Interface Science, 372 (2012) 183-191.
- Alvarez, N.J., L.M. Walker, and S.L. Anna, Low Concentration Dynamic Surface Tension Measurements of Macromolecular Systems. 2010: AIChE Annual Meeting, Salt Lake City, UT.
- N.J. Alvarez, S.L. Anna, T. Saigal, R.D. Tilton, L.M. Walker, Interfacial Dynamics and Rheology of Polymer-Grafted Nanoparticles at Air-Water and Xylene-Water interfaces. Langmuir, 28 (2012) 8052 – 8063.
- Jin, F., R. Balasubramaniam, and K.J. Stebe, Surfactant adsorption to spherical particles: The intrinsic length scale governing the shift from diffusion to kinetic-controlled mass transfer. Journal of Adhesion, 2004. 80(9): p. 773-796.
- Lin, S.Y., et al., Adsorption kinetics of C(12)E(8) at the air-water interface: Adsorption onto a clean interface. Langmuir, 1996. 12(26): p. 6530-6536.
- Lee, S., D.H. Kim, and D. Needham, Equilibrium and dynamic interfacial tension measurements at microscopic interfaces using a micropipet technique. 1. A new method for determination of interfacial tension. Langmuir, 2001. 17(18): p. 5537-5543.
- N.J. Alvarez, L.M. Walker, S.L. Anna, “A Criterion to Assess the Impact of Confined Volumes on Surfactant Transport to Liquid-Fluid Interfaces,” in press Soft Matter, June 21, 2012, DOI: 10.1039/c2sm25447f.
